The U.S. Food and Drug Administration (FDA) has granted Fast Track designation to Life Molecular Imaging’s (LMI) tau positron emission tomography (PET) imaging procedure. PI-2620.
The designation was granted for the clinical use of PI-2620 for tau PET imaging in Alzheimer’s disease (AD), progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD).
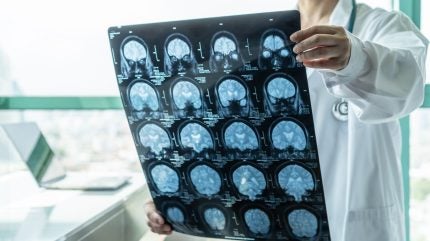
PI-2620 PET imaging is a technique in which the patient is injected with a small amount of the PI-2620 tracer, which then travels through the bloodstream and binds to any tau proteins in the brain.
Patients then undergo a PET scan, which detects the signals emitted by PI-2620. The resulting PET images show whether tau deposits are present in the brain and in which regions they are located. This helps diagnose and monitor the progression of AD and other neurodegenerative diseases in which tau deposits play a role.
PI-2620 is currently being investigated in a Phase III trial (NCT05641688) by LMI to evaluate the efficacy and safety of PET imaging with PI-2620 for the detection of tau deposits in patients with AD.
“This designation not only validates our approach but also facilitates closer collaboration with the FDA to accelerate the development of PI-2620,” said Andrew Stephens, CMO of LMI.
Access the most comprehensive company profiles on the market, powered by GlobalData. Save hours of research. Gain a competitive advantage.

Company profile – free sample
Your download email will arrive shortly
We are confident in the unique quality of our company profiles, but we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by filling out the form below.
By GlobalData
“We are committed to advancing this important imaging technique that has the potential to make a meaningful difference for patients who need accurate and accessible tau PET imaging.”
PI-2620 was developed in collaboration with AC Immune, a Swiss biotechnology company that focuses primarily on developing treatments for neurodegenerative diseases, but also collaborates with other companies and develops drug candidates through its Morphomer and SupraAntigen technology platforms.
Morphomer is capable of generating small molecules that bind to misfolded proteins such as amyloid beta (Aβ), while SupraAntigen generates monoclonal antibodies that specifically target misfolded proteins.
The FDA previously granted Fast Track designation to two of AC Immune’s immunotherapies, ACI-35-030 (NCT04445831) and ACI-24060. The compounds target tau and Aβ, respectively.
In January 2023, AC Immune reported positive data from the ABATE study (NCT05462106) of the anti-amyloid beta (Abeta) vaccine ACI-24060, finding that the compound elicited an anti-Abeta antibody response in the first low-dose cohort of AD patients at week six.
“The designation for PI-2620 is further recognition of AC Immune’s drug discovery and development platform and how we continue to drive innovation with our partners,” commented Andrea Pfeifer, CEO of AC Immune.
GlobalData’s pharmaceutical database shows that AC Immune has 20 drugs in active development, primarily for neurodegenerative diseases.
GlobalData is the parent company of Clinical trials arena.
The Alzheimer’s Association International Conference (AAIC) 2024 was held from July 28 to August 1 in Philadelphia, USA. During the conference, Eisai announced that patients treated continuously with the anti-amyloid therapy Leqembi for three years experienced a slowdown in the progression of Alzheimer’s disease. Leqembi received full marketing approval from the FDA in July 2023.


